ORIGINAL MATERIAL
ORIGINAL MATERIAL
ORIGINAL MATERIAL
We handle original cosmetic ingredients. It is the world's first new raw material born from advanced cell culture research.です。
iPS cell culture supernatant
iPS cells is a target of research in the regenerative medicine industry, though we have independently used the culture supernatant obtained when culturing iPS cells as a raw material for cosmetics. It is the world's first original raw material that has acquired the international standard INCI code and the Japanese sales name, that are names given to cosmetics ingredients,
【Expected effects】
・ Gives skin firmness and shine
・ Gives skin a transparent feel
・ Moisturizes the skin
・ Prevents rough skin
・ Gives hair firmness etc.
【Original Ingredient Name】
INCI name:Human Hepatocyte Induced Pluripotent Cell Culture Conditioned Media
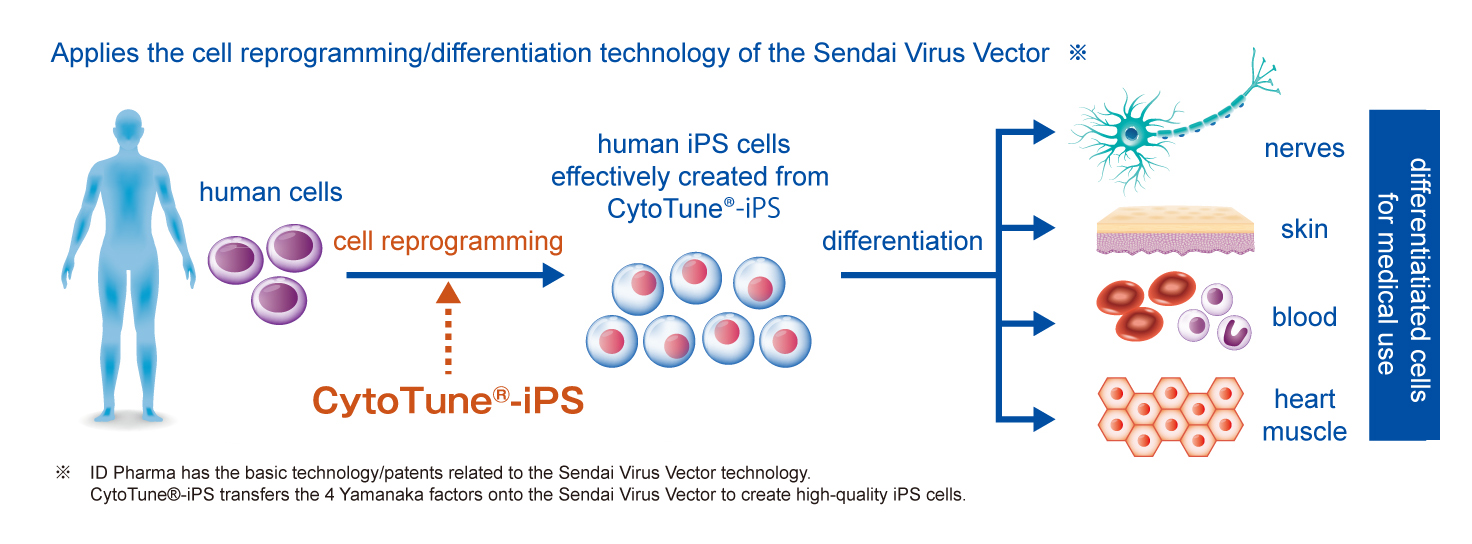
What are iPS cells? I’ROM Group’s iPS cell technology
What are iPS cells?
It is a type of an artificially created stem cell. It is made from "somatic cells" such as skin, liver, and blood. Unlike general stem cells, iPS cells have the ability to change (differentiate) into various tissues and proliferate indefinitely. There are no such stem cells other than iPS cells with such potential, except for ES cells that are derived from fertilized eggs.
I’ROM Group’s iPS cell technology
ID Pharma Co., Ltd., which manufactures this raw material, provides one of the global standard kits for producing iPS cells in the US, UK, France, China and other countries around the world. As a well-known company in the field of regenerative medicine, ID Pharma has greatly contributed to the development of this field.
In addition, it is co-developing various therapeutic agents and vaccines with public research institutes and universities by making the best use of advanced medical technology. Currently, the development of a new COVID-19 vaccine is ongoing, which has been featured in multiple media such as the NHK News Watch 9 and TV Asahi Hodo Station. Its progress is receiving attention from all over the world.